2B - Analysis IR /14

|
2B - Analysis IR /14 |
 |
For the following reaction:
A + B à C + H2O
| A | B | C | |
| Molecular formula | 37.50% C, 12.50% H and 50.00% O. Mr = 32 | 40.00% C, 6.67% H and 53.33% O. Mr = 60 | 48.65% C, 8.11% H and 43.24% O. Mr = 74 |
|
IR Spectra |
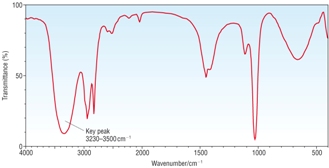 |
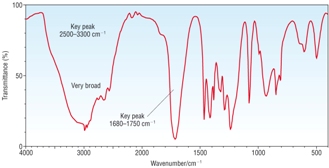 |
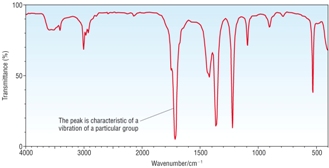 |
1) Identify A, B and C given the information above. [6]
2) Write a balanced equation for the reaction above. [1]
3) 1.60g of A reacted with 3.50g of B to make 3.50g of C.
Calculate the % yield for the reaction. [6]
4) Calculate the atom economy for the production of C [1]