2B - Analysis IR /14
|
2B - Analysis IR /14 |
|
For the following reaction:
A + B à C + H2O
| A | B | C | |
| Molecular formula | CH3OH [1] | CH3COOH [1] | CH3COOCH3 [1] |
|
IR Spectra |
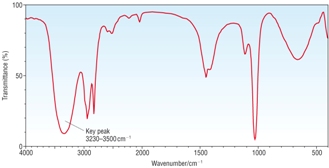 |
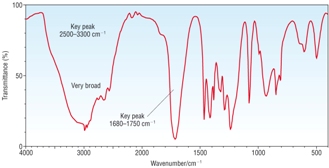 |
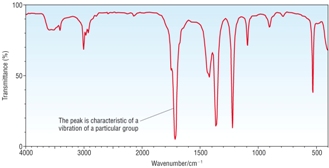 |
| OH peak only, no C=O peak [1] | OH peak and C=O peak [1] | C=O peak only, no OH peak [1] |
1) Identify A, B and C given the information above. [6]
A - Methanol B - Ethanoic acid C - Methyl Ethanoate
2) Write a balanced equation for the reaction above. [1]
CH3OH + CH3COOH à CH3COOCH3 + H2O
3) 1.60g of A reacted with 3.50g of B to make 3.50g of C.
Calculate the % yield for the reaction. [6]
Moles A / CH3OH = 0.0500 [1] Moles of B / CH3COOH = 0.0583 [1]
A / CH3OH is limiting reagent [1]
Moles C / CH3COOCH3 that could be made = 0.0500 [1]
Moles C / CH3COOCH3 that was actually made = 0.0473 [1]
% Yield = (0.0473 / 0.0500) x 100 = 94.6% [1]
4) Calculate the atom economy for the production of C [1]
Atom economy = 74 x 100 = 80.4%
(74+18)