2B - Review
|
2B - Review |
|
1. This question is about different models of bonding and molecular shapes. Magnesium sulfide shows ionic bonding.
(i) What is meant by the term ionic bonding? [1] (Electrostatic) attraction between oppositely charged ions
(ii) Draw a dot-and-cross diagram to show the bonding in magnesium sulfide. Show outer electron shells only. [2]
Mg shown with either 8 of 0 electrons AND S shown with 8
electrons with 2 crosses and 6 dots (or vice versa) ![]()
Correct charges on both ions ![]()

2. Dot-and-cross diagrams can be used to predict the shape of covalent molecules.
Fluorine has a covalent oxide called difluorine oxide, F2O. The oxygen atom is covalently bonded to each fluorine atom.
(i) Draw a dot-and-cross diagram of a molecule of F2O. Show outer electron shells only. [2]
Electron pairs in covalent bonds shown correctly using dots and crosses in a
molecule of the F2O ![]()
Lone pairs correct on O and both F atoms ![]()

(ii) Predict the bond angle in an F2O molecule. Explain your answer. [3]
Bond angle 104.5°. ![]() There are 2 bonded pairs and 2 lone pairs
There are 2 bonded pairs and 2 lone pairs ![]() Lone pairs repel more than bonded pairs
Lone pairs repel more than bonded pairs ![]()
3. Chemists have developed models for bonding and structure which are used to explain different properties.
Ammonia, NH3, is a covalent compound.
(i) Explain what is meant by a covalent bond. [1]
a
shared pair of electrons ![]()
(ii) Draw a dot-and-cross diagram to show the bonding in NH3. Show outer electrons only. [1]
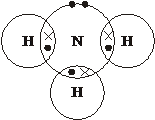
(iii) Name the shape of the ammonia molecule and explain, why ammonia has this shape and has a bond angle of 107°. [3]
pyramidal ![]() Explanation: There are 3 bonded pairs and 1 lone pair
Explanation: There are 3 bonded pairs and 1 lone pair ![]() Lone pairs repel more than bonded pairs
Lone pairs repel more than bonded pairs ![]()
4. Molecules of NCl3 have a bond angle of 107°.
(i) Name the shape of an NCl3 molecule.
[1]
pyramidal![]()
(ii) Explain why a molecule of NCl3 has this shape and a bond angle of 107°. [3]
electron pairs repel as far apart as possible ![]()
lone pairs repel more ![]()
4 electron pairs surround central atom or diagram with 3 bonds and a lone pair ![]()
5. Antimony is in Group 5 of the Periodic Table. It forms a compound with hydrogen that has the formula SbH3.
(i) Predict the bond angle in
SbH3.
[1]
Bond angle 107°. ![]()
(ii) Explain why a molecule of SbH3 has this bond angle. [2]
electron pairs repel electron pairs/bonds go as far apart as possible![]() lone
pairs repel more
lone
pairs repel more ![]()
6. The dot-and-cross diagram of an ammonia molecule is shown below.
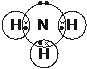
Predict, with reasons, the bond angle in an ammonia molecule. [4]
NH3:
107°. ![]() electron pairs repel other electron pairs
electron pairs repel other electron pairs ![]()
lone pair has more repulsion ![]() electron pairs get as far apart as possible
electron pairs get as far apart as possible ![]()