Ionisation energies and shells ![]()
1) Line emission spectra's
give a unique spectra to each elements. Explain how they arise. Use
the following terms in your answer: [4]
Excited
Emit
Energy
Light
Frequency
absorb Energy level
When electrons absorb energy are excited [1]
to a higher energy level [1] when they drop to a lower energy level
[1] the emit light [1]
at specific frequencies unique to that element
[1] any 4
2) The picture shows part of
a spectra for an element.
|
|
a) What do you notice about the
spectral lines as you move to the left? [1] The lines
converge / get closer together [1] b) Explain how this spectra has
changed the structure of the atom. [1]
The electron shells / energy levels get closer as you move away from the
nucleus [1] |
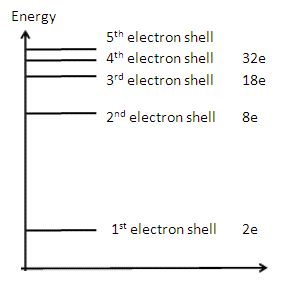
3) Sketch an energy level
diagram showing the first 3 principle quantum numbers and write on how many
electrons each energy level can hold. [4]
|
|
The energy levels
converge / get closer together [1] n = 1 has
2e [1] n = 2 has
8e [1] n = 3 has 18e [1] |
4) The following question is
about atomic orbitals:
a) What is an atomic orbital? [1] A region in space
around a nucleus where 2 electrons are likely to be found [1]
b)
What is the maximum amount of electrons in an orbital? [1] 2 electrons [1]
c)
How can 2 electrons occupy the same orbital when they both have a negative
charge? [1] They spin in opposite directions [1]
d)
What can you say about the electron density of an orbital containing 2
electrons rather than 1 electron? [1]
The electron density would be twice as much [1]