Mass spectroscopy and Ar calculations ![]()
1) A
sample of sulphur has three isotopes: sulphur-32, sulphur-33 and sulphur-34.
Calculate the relative atomic mass from the data below: Give your answer to the appropriate number of
decimals places.
|
percentage abundance |
95.02 |
0.76 |
4.22 |
|
Isotopic mass |
32 |
33 |
34 |
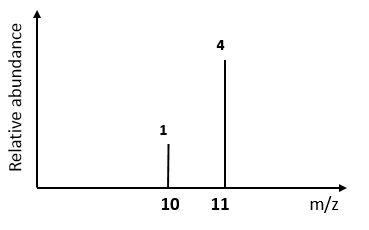
2) Boron has 2 isotopes and the
mass spectrum is shown below. Calculate
its relative atomic mass:
|
|
|
3) Lithium has two naturally occurring isotopes 6Li
and 7Li. Calculate the percentage abundance of each isotopes given
that the Ar of Lithium is 6.9. Show your working.
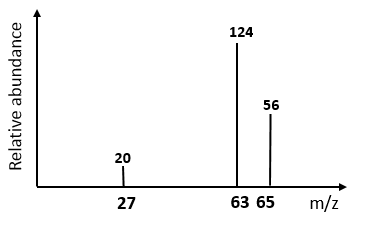
4) A specific alloy of bronze has been developed
for use in aircraft due to its high strength and resistance to corrosion.
It is an alloy of two metals and ts mass spectrum is shown below. Identify the two metals in
this bronze and explain why there are three peaks.
|
|
|
5) For the above question,
calculate the relative atomic mass of the element with Isotopes.
6) A
sample of magnesium has three isotopes: magnesium-24, magnesium -25 and
magnesium -26. Given that the relative atomic mass of magnesium is 24.3,
calculate the relative abundance for magnesium 24 from the data below:
|
Relative abundance |
|
100 |
110 |
|
Isotopic mass |
24 |
25 |
26 |
7) Sketch the mass spectrum you
would likely see for Chlorine molecules
given the following data:
|
Relative abundance |
3 |
1 |
|
Isotopic mass |
35 |
37 |
8) Mass spectroscopy is a
technique used to determine relative abundancies of isotopes of elements.
a) Briefly explain the stages in mass
spectroscopy:
b) Write an equation showing the ionisation
stage, use M to represent an atom:
c) Write an equation to show what happens at the
detector.