1 - Review all questions 2 answers /28
|
1 - Review all questions 2 answers /28 |
|
1) Name the following molecule: [1]
CH3CHBrCH2COOH
3 - bromo butanoic acid
2) Describe a simple chemical test to distinguish between an alkane and an alkene.
Include any observations. [3]
Add bromine water [1]
Stays orange with the alkane [1]
Turns clear and colourless with the alkene [1]
3) Show with the aid of curly arrows the mechanism for the reaction between hydrogen bromide and ethene [5]


Dipole on HCl [1]
Curly arrow from middle of C=C to d+ H [1]
Curly arrow from middle of H - Br to d- Br [1]
Correctly drawn carbocation [1]
Curly arrow from a pair of electrons on the Br ion to the positive carbon [1]
4) Describe the type of reagent and mechanism for question 9 [2]
Electrophilic [1] Addition [1]
5) Write an equation showing the initiation step for the reaction between chlorine and methane.
Describe the bond breaking. [2]
Cl2 à Cl. + Cl. UV light [1]
Homolytic fission [1]
6) For the reaction in (4), write out the propagation steps [2]
Cl. + C2H6 à HCl + .C2H5 [1]
.C2H5 + Cl2 à C2H5Cl + Cl. [1]
7) For the reaction in (4), write out the termination steps [3]
8) Describe the type of reagent and mechanism for questions 4 - 6 [2]
Free radical [1] Substitution [1]
9) Describe how you would convert propene to propane [3]
Hydrogen [1]
Ni catalyst [1]
150oC [1]
10) Describe how you would convert ethane to propanol [2]
Steam [1]
H3PO4 / phosphoric acid [1]
11) What type of reaction would convert hexane into benzene [1]
Reforming [1]
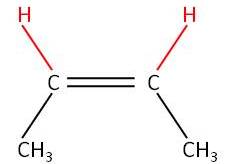
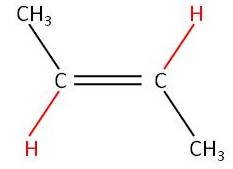
12) Draw and label the stereoisomers of but - 2 - ene [2]
 |
 |
|
Cis
but-2-ene (Z-but-2-ene) [1] |
Trans
but-2-ene (E-but-2-ene) [1] |