Group 2 elements: Redox eactions:
The Group 2 elements:
These are also called the alkaline earth metals as their hydroxides are alkaline.
Remember that the reactivity increases as you move down Group 2 (see ionisation energies)
Physical properties:
All light metals.
Compounds are white or colourless.
They have reasonably high melting and boiling points.
Electronic configuration:
All in the s block sub - shell and all have s2 electrons.
Reactivity of Group 2 elements:
Group 2 metals are reactive:
| M | à | M2+ | + | 2e- |
These elements give away 2 electrons when they react.
This means that what ever they react with must gain electrons.
Gaining electrons is a reduction reaction:
Oxidation
Is
Loss of electrons
Reduction
Is
Gain of electrons
As the Group 2 elements cause the reduction of other compounds or elements we say it is a good Reducing agent.
Reactivity increases as you go down the Group. This means they lose their electrons more readily.
This means as you go down Group 2, they become better Reducing agents
Reaction with oxygen
Group 2 metals react vigorously with oxygen to give the oxide:
Metal + Oxygen à Metal oxide
The reaction gives an ionic product. If you apply oxidation numbers, you can see what has been oxidised and reduced:
| Calcium | + | Oxygen | à | Calcium oxide |
| 2Ca(s) | + | O2(g) | à | 2CaO(s) |
| Element | 2Ca(s) | + | O2(g) | à | 2CaO(s) | Change in Ox No | Redox | |
| Up | Down | |||||||
| Ca | 0 | +2 | 2 | Ox | ||||
| O | 0 | - 2 | 2 | Red | ||||
Reaction with water
Group 2 metals react with water to give the hydroxide and hydrogen gas:
Metal + Water à Metal hydroxide + Hydrogen
The reaction gives an ionic product. If you apply oxidation numbers, you can see what has been oxidised and reduced:
| Calcium | + | Water | à | Calcium hydroxide | + | Hydrogen |
| Ca(s) | + | 2H2O(l) | à | Ca(OH)2(aq) | + | H2(g) |
| Element | Ca(s) | + | 2H2O(l) | à | Ca(OH)2(aq) | + | H2(g) | Change in Ox No | Redox | |
| Up | Down | |||||||||
| Ca | 0 | +2 | 2 | Ox | ||||||
| H | +1 | (+1) | 0 | 1 | Red | |||||
| O | -2 | -2 | - | - | - | - | ||||
Questions 1 - 2 P89
Group 2 oxides and hydroxides:
These are bases as they neutralise acids to form salts and water:
| MgO(s) | + | 2HCl(aq) | à | MgCl2(aq) | + | H2O(l) |
| Ca(OH)(s) | + | 2HCl(aq) | à | CaCl2(aq) | + | 2H2O(l) |
1) Group 2 oxides:
These react with water to form metal hydroxide.
| MgO(s) | + | H2O(l) | à | Mg(OH)2(aq) |
These are soluble so dissolve in the water forming an alkali, pH10 - 12
2) Group 2 hydroxides:
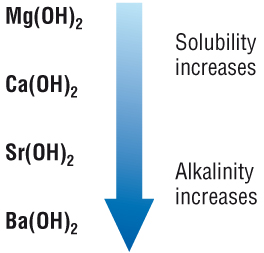
As these are soluble, they dissolve in water forming alkaline solutions::
| Ca(OH)2(s) | + | aq | à | Ca2+(aq) | + | 2OH-(aq |
 |
|
3) Group 2 carbonates:
The Group 2 carbonates decompose when heated, Thermal Decomposition:
Is the breaking down of a chemical substance with heat into at least 2 chemical substances
| MgCO3(s) | à | MgO(s) | + | CO2(g) |
 |
|
Properties of Group 2 elements and their compounds:
Because of Periodicity we only have to learn the Chemistry for one of the elements in Group 2.
All the elements in Group 2 will react in the same way (but with different vigour).
As you go down Group 2: the elements become more reactive.
As you go down Group 2: the carbonates decompose at higher temperatures.
As you go down Group 2: the hydroxides become more soluble in water, making the solution more alkaline.
Reactions of calcium and its compounds (or any Group 2 element / compound)

Uses of Group 2 hydroxides:
1) Calcium hydroxide
Is used to reduce the acidity of soil:
| Ca(OH)2(s) | + | 2HCl(aq) | à | CaCl2(aq) | + | 2H2O(l) |
2) Magnesium hydroxide
Is used in indigestion tablets / 'milk of magnesia' to neutralise excess stomach acid.
| Mg(OH)2(s) | + | 2HCl(aq) | à | MgCl2(aq) | + | 2H2O(l) |
Questions 1 - 2 P91 / 4, 9, 11, and 12 P97 / 4 P98