2C - Bonding and structure
|
2C - Bonding and structure |
|
1) This question is about copper:
a) Describe metallic bonding. You may wish to use a diagram. [2]
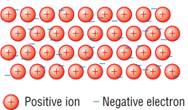
Labelled diagram [1] electrostatic forces of attraction between delocalised electrons and metal ions [1]
b) How does a metal conduct electricity? [1]
The electrons are free to move [1]
2) This question is about the salt sodium chloride, NaCl:
a) Discuss the conductivity of NaCl compounds. Explain your answer. [5]
Solids do not conduct [1] as the ions are held in a fixed position [1]
Dissolved [1] and molten [1] as the ions are free to move [1]
b) With the aid of a diagram explain the solubility of sodium chloride in water. [4]
Water is a polar molecule [1]
These are attracted to the ions, weaken the ionic bonds pulling them away from the lattice [1]
3) Complete the following table for the following substances: [20]
| NaCl | SiO2 | Br2 | NH3 | ||
| Structure | Giant Ionic | Giant covalent | Simple covalent | Simple covalent | |
| Melting point | High | High | Low | Low | |
| Electrical conductivity | aq / molten | No | No | No | |
| Solubility in water | Yes | No | No | Yes | |
| Solubility in non polar solvent | No | No | Yes | No |
Each box correct [1] x 20