2A - Electrons - review
|
2A - Electrons - review |
|
1) Define the term first ionisation energy. [3]
the energy required to remove one electron ![]() from
each atom in one mole
from
each atom in one mole ![]() of
gaseous atoms
of
gaseous atoms ![]()
2) Electrons are arranged in energy levels.
(a) An orbital is a region in which an electron may be found. Draw diagrams to show the shape of an s orbital and of a p orbital. [2]

(b) Complete the table below to show how many electrons completely fill each of the following. [3]
|
|
number of electrons |
|
a d orbital |
2 |
|
a p sub-shell |
6 |
|
the third shell (n = 3) |
18 |
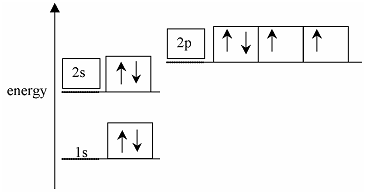
3) The energy diagram below is for the eight electrons in an oxygen atom. The diagram is incomplete as it only shows the two electrons in the 1s level.
Complete the diagram for the oxygen atom by:
(a) adding labels for the other sub-shell levels, [1]
(b) adding arrows to show how the other electrons are arranged. [1]
4) The table shows the first 6 successive ionisation energies of an element X, which is in Period 3 of the Periodic Table.
Use the table to identify element X. Explain how you decided on your answer. [3]
|
element |
ionisation energy / kJ mol–1 |
|||||
|
|
1st |
2nd |
3rd |
4th |
5th |
6th |
|
X |
578 |
1817 |
2745 |
11 578 |
14 831 |
18 378 |
Al![]() Sharp rise in successive ionisation energy between 3rd and 4th IE
Sharp rise in successive ionisation energy between 3rd and 4th IE![]() marking a change to a new or different shell / there are 3 electrons in the
outer shell
marking a change to a new or different shell / there are 3 electrons in the
outer shell ![]()
5) (a) Write an equation, with state symbols, to represent the second ionisation energy of calcium. [2]
Ca+(g)
®
Ca2+(g)
+
e−
Equation with correct charges and 1 electron lost ![]() state symbols
state symbols ![]()
(b) Why is the second ionisation energy of calcium greater than their first ionisation energies? [1]
same number of protons or same nuclear charge attracting less electrons /
electron removed from an ion /
ion is smaller![]()
(c) Explain why the first ionisation energy of strontium are less than that of calcium. [3]
atomic radii of Sr > atomic radii of Ca / Sr has electrons in shell further from
nucleus than Ca / Sr has electrons in a higher energy level / Sr has more shells ![]()
Therefore less attraction
Sr has more shielding than Ca
(‘more’ is essential)
increased nuclear charge is outweighed / despite increased nuclear
charge …..by at least one of the factors above
6) The electronic configuration of a bromine atom can be written in terms of sub-shells.
(a) Complete the electronic configuration of a bromine atom. [2]
1s22s22p63s23p6 ...............
1s22s22p63s23p6..........3d104s24p5 ![]()
![]() Award 1 mark for p5.
Award 1 mark for p5.
(b) Why is bromine classified as a p-block element? [1]
Highest energy sub-shell/sub-shell/being filled is the p sub-shell/outer
electrons are in a p (sub-shell/orbital/shell) ![]()
(c) Write the electron configuration for a bromine ion [1]
1s22s22p63s23p6 ...............
1s22s22p63s23p6..........3d104s24p6 ![]()