Review all
|
Review all |
| Target revision in: | ||||
| 1 | Draw an oxide ion (3) |
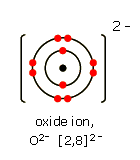 |
Nucleus with 8p and 8n = 1 electron shells = 1 Brackets and 2- = 1 |
Drawing atoms and ions |
| 2 | What does 37Cl and 35Cl have in common? (2) 17 protons (1) and 17 electrons (1) | Isotopes and the definition | ||
| 3 | How does 37Cl and 35Cl differ? (1) 20 Neutrons and 18 neutrons (1) | Isotopes and the definition | ||
| 4 | Write a balanced chemical reaction for Aluminium and Sulphuric acid (4) | Formula, acid reactions and balancing | ||
| 2Al + 3H2SO4 à Al2(SO4)3 + 3H2 | ||||
| H2SO4 = 1 Al2(SO4)3 = 1 H2 = 1 Balanced = 1 | ||||
| 5 | Assign Oxidation numbers to determine what has been reduced in the reaction above, explain your answer (6) | |||
| 2Al + 3H2SO4 à Al2(SO4)3 + 3H2 | Ox no's and REDOX | |||
| Al = 0
H = 1+ S = 6+ O = 2-
Al = 3+ S = 6+ O = 2-
H = 0 1 mark for each element correct on both sides of the
arrow Hydrogen has been reduced as its Ox No has reduced / gained electrons |
||||
| 6 | What is the reducing agent? (1) Al | Ox and Red agents | ||
| 7 | How would you define reduction (1) gain of electrons | OIL RIG | ||
| 8 | Calculate the % of S in H2SO4 (2) (32.1/98.1) x 100 (1) = 32.7 (3sf) (1) | % element in a molecule | ||
| 9 | Sulphuric acid is a strong acid, explain what this means? (1) Dissociates completely | Acids definitions | ||
| 10 | Sulphuric acid contains 2 protons, what do we call acids that contain 2 protons (1) Diprotic acids | |||
| 11 | Sulphuric acid can be neutralised by alkalis, what is an alkali? (1) a species when dissolved in water releases hydroxide ions, OH 1- | Alkali definition | ||
| 12 | Look at this reaction: | |||
| Br2 | + | H2S | à | 2HBr | + | S | |
| Br = 0 | H = 1+ S = 2- | H = 1+ Br = 1- | 0 |
| a | Assign oxidation numbers to each element (3) 1 mark for each element correct on both sides of the arrow | |
| b | What is the spectator ion ? Explain your answer (2) H+ (1) as there is no change in the Ox No | Ox no's and spectator ions |
| c | Rewrite the reaction as an ionic equation (2) S2- = 1 and 2Br 1- = 1 | ionic equations |
| Br2 | + | S2- | à | 2Br 1- | + | S |