3BP (areas)
4BP
3BP / 1LP
2BP / 2LP
2 Areas (4BP)
6BP
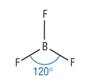
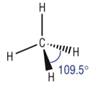
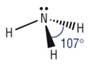
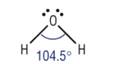
Trigonal planar
Tetrahedral
Pyramidal
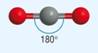
Non - linear
Linear
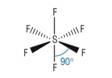
Octahedral
Definitions and standard answers:
Standard answers – Module 1A
1) Isotope: Same number of protons and electrons, different number of neutrons
2) Acid: Proton donor
3) Base: proton acceptors
4) Strong acid: Dissociates fully in water
5) Weak acid: Partially dissociates in water
6) Salt: When the H+ ion in an acid is replaced with a metal (ammonium) ion
7) Alkali: Dissociate to give hydroxide ions in water
8) Acid reactions:
Metal + acid à Salt + hydrogen
Metal oxide + acid à Salt + water
Metal hydroxide + acid à Salt + water
Metal carbonate + acid à Salt + water = carbon dioxide
9) % element = No of that element x Ar x 100
Mr
10) Oxidation is loss of electrons
11) Reduction is gain of electrons
Standard answers – Module 1B
1) Relative isotopic mass: mass of an atom of an isotope compared with 1/12 of the mass of an atom of 12C
2) Relative atomic mass, Ar: weighted mean mass of an atom of an element compared with 1/12 of the mass of an atom of 12C
3) Relative molecular mass, Mr: weighted mean mass of an molecule compared with 1/12 of the mass of an atom of 12C
4) Relative formula mass, Mr: weighted mean mass of a formula unit compared with 1/12 of the mass of an atom of 12C
5) RAM = (% x Ar) + (% x Ar)
100
6) Empirical formula: simplest whole number ratio of atoms of each element in a compound
7) Molecular formula: actual whole number ratio of atoms of each element in a compound
8) Moles (s) = mass (g)
Mr
(Aq) = Conc (mol dm-3) x Vol (dm3)
(g) = Vol (dm3)
24 (dm3)
Standard answers – Module 2A
1) First ionisation energy: energy required to remove 1 electron from each atom in a mole of gaseous atoms to form 1 mole of gaseous 1+ ions
2) Atomic orbital: region in space around the nucleus that can contain 2 electrons with opposite spins
Standard answers – Module 2B
1) Ionic bonding: Electrostatic force of attraction between oppositely charged ions
2) Covalent bond: Formed by a pair of shared electrons
3) Dative covalent bond: Formed by a pair of shared electrons where both electrons are provided by one atom
4) What determines the shape of a molecule:
· Pairs of electrons repel as far as possible
· This determines the shape
· Lone pairs repel more than bonding pairs as closer to central atom
· Each lone pair reducing the bond angle by 2.5o as it is closer to the central atom
|
3BP (areas) |
4BP |
3BP / 1LP |
2BP / 2LP |
2 Areas (4BP) |
6BP |
|
|
|
|
|
|
|
|
Trigonal planar |
Tetrahedral |
Pyramidal |
Non - linear |
Linear |
Octahedral |
5) How does the addition of H+ ion change shape of ammonia (water)
|
3BP / 1LP |
|
4BP |
|
|
The addition of a datively bonded H+ ion uses the lone pair converting it to a bonding pair (similar for water) |
|
|
Trigonal planar |
|
Tetrahedral |
Standard answers – Module 2C
1) Electronegativity: The power of an atom to attract bonding pairs of electrons towards itself
2) IMF – VDW: between atoms of similar electronegativity
· Uneven distribution of electrons
· Instantaneous dipole
· Induced dipole
· Weak force of attraction
3) IMF – Permanent dipole – dipole forces of attraction:
|
|
|
Is between molecules with a permanent dipole |
4) IMF – Hydrogen bonding:
|
|
· Dipoles · O,N,F bonded to H · LP on O,N,F · Dotted line between LP and H · Labelled
|
5) Anomalous properties of water:
· Unusually high melting / boiling point – H bonding is strongest IMF, more energy needed to overcome
· Ice less dense than water – H bonds are longer than covalent bonds
· Surface tension – extensive H bonds across the surface of water
6) Metallic bonding:
|
|
· LABELLED DIAGRAM · Electrostatic forces of attraction between metal ions and delocalised electrons |
7) Conductivity of metals:
· Electrons are free to move
8) Conductivity of ionic compounds:
· Solid – does not conduct as ions in a fixed position
· Molten / dissolved – does conduct electricity as ions are free to move
Standard answers – Module 3A
1) Explain the trend in boiling point of Gp 1 – 3 metals:
· As you go across Period, metal ions have a larger charge
· Also has more delocalised electrons
· Attraction is greater between larger ionic charge and more delocalised electrons
2) State and explain the trend in atomic radii across a Period:
· Across period nuclear charge increases
· Electrons in same shell, shielding remains the same
· Greater attraction
· Atomic radius decreases
3) State and explain the trend in first ionisation energies across a Period:
· Across period nuclear charge increases
· Electrons in same shell, shielding remains the same
· Greater attraction
· Atomic radius decreases
· Electrons more difficult to remove
4) State and explain the trend in Atomic radii down a Group:
· More electron shells
· Atomic radii increases
5) State and explain the trend in ionisation energies down a Group:
· More electron shells
· More shielding
· Atomic radii increases
· Attraction decreases despite an increase in the nuclear charge
6) State and explain the trend in melting / boiling points across a period:
Gp 1-3:
· Increases
· Metal ions have a larger charge
· Also has more delocalised electrons
· Attraction is greater between larger ionic charge and more delocalised electrons
Gp 4:
· Highest
· Giant covalent structure, extensive strong covalent bonds
· Lots of energy required to break covalent bonds
Gp 5-7:
· Low
· Simple molecular – weak IMF – VDW
· Little energy required
Gp 0:
· Lowest
· Atomic – weak IMF – VDW
· Little energy required
Standard answers – Module 3B
1) State and explain the reactivity as you go down Gp 2:
· Reactivity increases down group
· Atomic radius increases due to more shells
· More inner shells, shielding
· Overall attraction decreases
· Easier to remove outer electrons
2) State and explain the alkalinity / solubility of Gp 2 hydroxides:
· Solubility increases down the group
· More hydroxide ions released
· M(OH)2(s) + aq à M2+(aq) + 2OH-(aq)
· More hydroxides = more alkaline
3) State the ease of the decomposition of Gp 2 carbonates:
· Ease of decomposition decreases down the group
Standard answers – Module 3C
1) State and explain the trend in the boiling points down Gp7:
· Increases as you down the group
· Due to greater number of electrons
· Causing stronger VDW forces of attraction
2) Colours of the halogens
|
|
Cl2 |
Br2 |
I2 |
|
Aq |
pale green |
orange |
brown |
|
Solvent |
Pale green |
orange |
purple |
3) Precipitation reactions of the halides
|
|
Cl- |
Br- |
I- |
|
AgNO3 |
White ppt |
Cream ppt |
Yellow ppt |
|
Ammonia |
Dissolves in dil |
Dissolves in conc |
Insoluble |
4) State and explain the reactivity as you go down Gp 7:
· Reactivity decreases down group
· Atomic radius increases due to more shells
· More inner shells, shielding
· Overall attraction decreases
· Harder to capture an electron
4) Disproportionation: Has been both oxidised and reduced