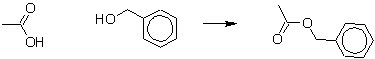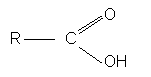
Carboxylic acids have the general formula:

These are present in many foods:
Ethanoic acid – vinegar
Benzoic acid – used as flavouring in lemonade
Citric acid – Used as flavouring in citrus drinks
Esters have the general formula:
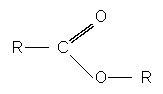
Esters are referred to as a derivative of carboxylic acids due to the easy substitution of the hydrogen in the hydroxyl group with an alkyl group. They are derived from carboxylic acids.
Again these are found in foods and have a fruity aroma.
Natural
Naptha from crude oil.
Natural oils.
Oils and fats are esters of carboxylic acids. Hydrolysis provides the carboxylic acids.
Carboxylic acids from oils and fats usually have long carbon chains and are often referred to as fatty acids.
They usually contain an even number of carbon atoms and are not branched.
If a fatty acid contains 1 C=C, we call it monounsaturated.
With more than 1 C=C, we call them polyunsaturated. Each double bond will give rise to geometric isomers.
Synthetic
From alcohols, aldehydes and nitriles
SAQ 4.1 and 4.2
EXPT – The reactions of carboxylic acids
1) Solubility and pH
Add 1cm3 of pure ethanoic acid (called glacial) to a test tube (CARE very corrosive).
Add water dropwise with a pipette, do the liquids mix?
Spot out some of the mixture on to full range indicator paper.
Now add a few drops of sodium carbonate solution.
See how well other carboxylic acids mix with water
Record all observations.
2) As an acid
a) With sodium hydroxide
Set up apparatus for titration using a small beaker.
Add 25cm3 of 0.1M ethanoic acid to a small beaker add a pH probe and place on a magnetic stirrer.
Make sure to pH probe will not be hit by the stirrer.
Fill a burette with 0.1M sodium hydroxide.
Start logging and run all of the sodium hydroxide into the ethanoic acid.
Sketch the graph.
b) With magnesium
Add 10cm3 of ethanoic acid to a test tube.
Add about a 3cm strip of magnesium ribbon andplace a boiling tube over the top.
After a minute, test the gas with a lit splint.
Record all observations.
c) With sodium carbonate
Add 5cm3 of sodium carbonate solution to a test tube.
Add ethanoic acid dropwise to the solution
Record all observations.
3) Formation of esters
Add 1cm3 of pure ethanoic acid to a test tube.
Add 2 cm3 of ethanol.
Add 2 – 3 drops of concentrated Sulphuric acid.
Heat in a water bath for about 5 minutes.
Add a few drops of sodium carbonate solution until it no longer fizzes. This should neutralise any excess acid.
Smell the product; compare it to the smell of ethanoic acid.
You can repeat this using different alcohols.
Interpretation of the reactions of carboxylic acid
In chapter 2 we looked at the behaviour of the hydroxyl group in phenol and had to modify its behaviour to take into account the interaction of the lone pair of electrons from the oxygen atom into the benzene ring.
Again we must look at the properties and modify if necessary to take onto account the interaction of the lone pair of electrons from the oxygen atom and the double bond of the carbonyl group.
1) Solubility and pH
Solubility – C1 - C4 carboxylic acids mix readily.
C5 à solubility reduces due to length of insoluble R chain.
All carboxylic acids H - bond to each other and with water.
Carboxylic acids are weak in water.
Less than 1% ionized:-
RCO2H(aq) à H+(aq) + RCO2-(aq)
Methanoic acid (formic acid) is used in stings by some insects.
Electron diffraction studies show that the C – O bond lengths in the methanoate ion are equivalent.
The C – O bonds lengths are shorter than the C – O single bond, but longer than the C =O double bonds.
This is evidence of delocalisation of the p bond electrons of the carbonyl group (C = O) with the C – O group of the hydroxyl group:-
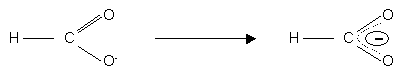
The proximity of the carbonyl group allows the O – H bond to partially ionise in water.
When hydroxyl group ionises, the anion is stabilised by delocalisation of the C = O p electrons.
Because of this we would expect some acidic behaviour:-
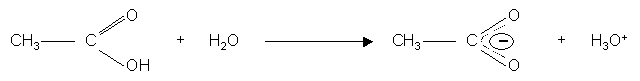
Carboxylic acids are acidic enough to react with:
Metals
Metal oxides
Metal hydroxides
Metal carbonates
a) With sodium hydroxide
CH3CO2H + NaOH à CH3CO2Na + H2O
b) With magnesium
2CH3CO2H + Mg à (CH3CO2)2Mg + H2
c) With sodium carbonate
2CH3CO2H + Na2CO3 à 2CH3CO2Na + H2O + CO2
In each case the carboxylic acid has donated a proton, H+
A salt is formed each time
Dipole due to C = O group means the carbon atom is electrophylic (d+).
This means carboxylic acids will be susceptible to nucleaphilic attack:-
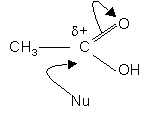
Even weak nucleophiles (e.g. ethanol) will attack the carbon atom but require an acid catalyst.
Carboxylic acids react with alcohols to form esters and water:-
Carboxylic + Alcohol à ester + water
acid
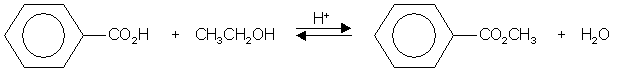
An acid catalyst (sulphuric acid) + heat is required.
SAQ 4.3 and 4.4
The derivatives of carboxylic acids are those where the – OH group in the – CO2H functional group has been replaced with another group:

In the carboxylic acid section it was possible to make an ester using an alcohol.
Hydrolysis of any ester will make the corresponding carboxylic acid (and alcohol):
Ester + water à Carboxylic + Alcohol
acid
Compare this reaction with how an ester was made:
Carboxylic + Alcohol à ester + water
acid
You should notice that they are reverse reactions of each other.
1) Add 2cm3 of methyl benzoate and 30cm3 of 2M sodium hydroxide to a 50cm3 pear shaped flask. Add a couple of anti bumping granuals.
2) Assemble the apparatus for reflux and reflux for 30 minutes.
3) Allow the mixture to cool then acidify with 2M hydrochloric acid. Spot onto universal indicator paper to check.
4) Write a balanced chemical reaction to show what has happened.
OPTIONAL – If time allows
5) Collect your product by suction filtration and wash with some distilled water.
6) Recrystalise the product with water and determine its melting point.
7) Use the data book to check purity.
SAQ 4.5
The hydrolysis of any carboxylic acid derivative results in the carboxylic acid.
The reaction undergoes nucleophilic substitution:-
Hydrolysis of an ester
With acid catalyst
|
|
The reaction is in equilibrium which means molecules of reactants and products are present in the reaction mixture.
With Base catalyst
When a base is used, the product is the sodium salt of the carboxylic acid:
|
|
SAQ4.6
|
Propane 1,2,3 triol (glycerol) |
+ |
|
|
|
||
|
|
||
|
Octadecanoic acid (stearic acid) |
|
|
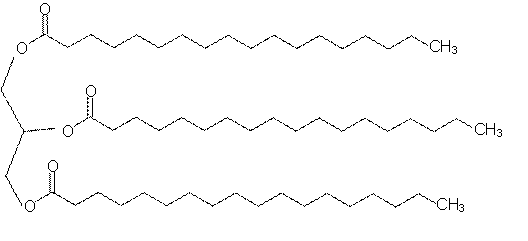
A triglyceride
Glycerol has 3 alcohol groups which can react with a carboxylic acid (stearic acid) to form an ester. This is called esterification.
The carboxylic acids occur naturally and because they make fats (upon esterification) we call them fatty acids.
If all 3 join we make a triglyceride (an ester). These are fats and oils and occur naturally:
If only one of the alcohol groups is esterified with 1 stearic acid we make a mono glyceride, with 2, diglyceride.
A triglyceride (fat or oil) actually looks like:
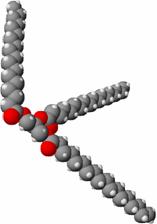
SAQ 4.7
The hydrolysis of fats and oils - saponification
Oils are basically just esters. This means they can be hydrolysed like any other ester by refluxing with an acid or base catalyst.
An acid catalyst gives glycerol and the fatty acids (carboxylic acids eg stearic acid).
With a base you make glycerol and the sodium salts of the fatty acids:

3NaOH +
|
|
|
Propane 1,2,3 triol (glycerol) |
+ |
|
|
|
|
Sodium Octadecanoate (Sodium stearate) The salt of a fatty acid |
|
|||
The salts of fatty acids are actually soaps.
The Latin for soap is ‘sapo’ hence this reaction is called saponification.
After saponification the soap is precipitated out by adding an excess of sodium chloride. This is known as ‘salting out’.
Solvents
Adhesives
Nail varnish remover
White board pens
Food flavorings
Essential oils
Essential oils
The odours from flowers come from volatile organic compounds.
These are extracted by steam distillation or made synthetically
They are usually made up from a mixture of asters, aldehydes, phenols and terpenes (a 5 carbon unit molecule).
Oil of jasmine is extracted from the jasmine plant. Alternatively it can be synthesised by the esterification of ethanoic acid and phenyl methanol: