1B Carbonyls /23
|
1B Carbonyls /23 |
|
1) Draw the displayed formula for 2 - methyl pentanal [1]
.jpg)
2) State and explain the bonding in the carbonyl group. You may wish to include a diagram: [3]
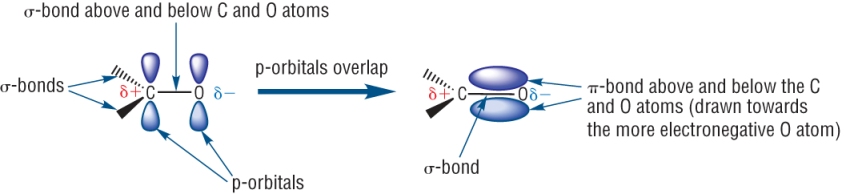
diagram and p orbitals labelled [1]
diagram and p orbitals labelled [1]
p orbitals overlap forming p orbitals [1]
3) Name and draw the structure of the product(s) formed when 2 methyl propanoic acid is treated with NaBH4: [4]
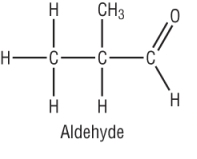 |
2 methyl propanal
(shown) [1] drawn [1] 2 methyl propan - 2 - ol [1] drawn [1] |
4) Complete the mechanism for the reduction of this aldehyde: [6]
|
|
Dipole on carbonyl [1]
Curly arrow from LP on hydride to the d+ carbon in the carbonyl [1] Curly arrow from middle of the C=O bond to the O [1] Correct intermediate drawn [1] Curly arrow from the LP on the O to the d+ H in water [1] Curly arrow from the middle of the H - O bond in water to the O in water [1] |
5) Describe a simple chemical test to distinguish between an aldehyde and a ketone: [4]
| Add sodium
dichromate [1] and sulphuric acid [1] Orange to green with aldehyde [1] No change with a ketone [1]
|
Add silver nitrate [1]
dissolved in ammonia [1] Silver mirror / precipiatate with aldehyde [1] No change with a ketone [1] |
6) Outline a chemical process to determine which aldehyde or ketone you have: [5]
| Add 2,4,DNPH [1] you get
orange crystals [1] Filter, recrystalise, filter and dry [1] Carry out a melting point determination [1] Compare melting point with known data [1]
|