1B Carboxylic acids /12
|
1B Carboxylic acids /12 |
|
1) a) Write the molecular formula for ethyl propanoate [1]
CH3CH2COOCH2CH3
b) Write a balanced chemical equation for the formation of ethyl propanoate [1]
CH3CH2COOH + HOCH2CH3 à CH3CH2COOCH2CH3 + H2O
c) Write a balanced chemical equation for the reaction between ethanoic acid and sodium metal [1]
CH3COOH + Na à CH3COO-Na+ + 1/2 H2
d) Write a balanced chemical equation for the reaction between ethanoic acid and potassium hydroxide [1]
CH3COOH + KOH à CH3COO-K+ + H2O
e) Write a balanced chemical equation for the reaction between ethanoic acid and calcium carbonate [1]
2CH3COOH + CaCO3 à (CH3COO-)2Ca2+ + H2O + CO2
2) Esterification can be reversed by hydrolysis. Write equations for the hydrolysis of:
a) Acid hydrolysis of Methyl propanoate [1]
CH3CH2COOCH3 + H2O à CH3CH2COOH + HOCH3
b) Acid hydrolysis of Ethyl methanoate [1]
HCOOCH2CH3 + H2O à HCOOH + HOCH2CH3
c) Alkaline hydrolysis (NaOH) of Propyl ethanoate [1]
CH3COOCH2CH2CH3 + NaOH à CH3COO-Na+ + HOCH2CH2CH3
d) Alkaline hydrolysis (NaOH) of Methyl ethanoate [1]
CH3COOCH3 + NaOH à CH3COO-Na+ + HOCH3
3) Ethanoic anhydride is much more reactive than ethanoic acid.
a) Draw the structure of ethanoic anydride and explain how it is formed: [1]
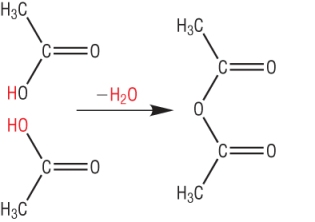
b) Write an equation for the formation of methyl ethanoate using ethanoic anyhdride [2]

Methyl ethanoate as product [1]
Rest of the equation [1]